A Lesson on Spot Free Water using Reverse Osmosis

The separation of dissolved solids and water using Reverse Osmosis membranes is a pressure driven temperature dependent process. The membrane material is designed to be as permeable to water as possible, while maintaining the ability to reject dissolved solids.
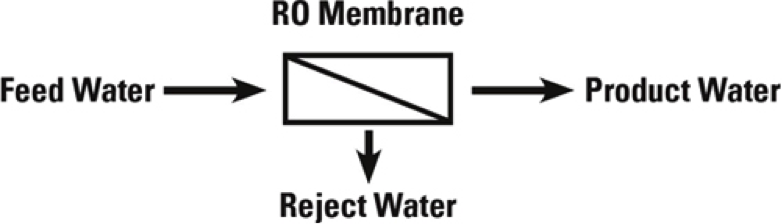
Reverse Osmosis systems utilize semi-permeable membrane elements to separate the feed water into two streams. The pressurized feed water is separated into purified (product) water and concentrate (reject) water. The impurities contained in the feed water are carried to drain by the reject water. It is critical to maintain adequate reject flow in order to prevent membrane scaling and/or fouling.
Reverse Osmosis refers to a process of water purification that has been used primarily for the desalination of seawater. To understand RO, it is first necessary to understand osmosis. Osmosis is the term for the phenomenon whereby if a semi-permeable membrane separates two salt solutions of different concentration, water will migrate from the weaker solution through the membrane to the stronger solution, until the solutions are of the same salt concentration. Reverse Osmosis subverts this process. It involves applying pressure to reverse the natural flow of water, forcing the water to move from the more concentrated solution to the weaker. The semi-permeable membrane is porous, allowing water to pass through, but blocking the passage of the bulkier salt .
The main system design parameters require the following:
- Internal flows across the membrane surface must be high enough to prevent settling of fine suspended solids on the membrane surface.
- The concentration of each dissolved ionic species must not exceed the limits of solubility anywhere in the system.
- Pre-treatment must be sufficient to eliminate chemicals that would attack the membrane materials.
Reverse osmosis has been used as a method of purification for ground and surface fresh water, in addition to its role as a desalinating agent. Working with such water sources creates some problems for the reverse osmosis system. Because of the very small pore sizes involved in the membrane, it is vital that ground and surface water is adequately pre-treated prior to the reverse osmosis process. Depending upon the hardness of the water involved, scaling of the membrane is likely to occur. If the concentration of the calcium or magnesium in the water (the chemicals that determine water’s hardness) is at a high enough level where the chemicals are insoluble, it will create a hard mineral on the inside of the membrane, rendering it impotent.
Pre-Treatment
The RO feed water must be pretreated in order to prevent membrane damage and/or fouling. Proper pretreatment is essential for reliable operation of any RO system.
Pretreatment requirements vary depending on the nature of the feed water. Pretreatment equipment is sold separately. The most common forms of pretreatment are described below.
Media Filter: Used to remove large suspended solids (sediment) from the feed water.
Backwashing the media removes the trapped particles. Backwash can be initiated by time or differential pressure.
Water Softener: Used to remove calcium and magnesium hardness from the feed water in order to prevent hardness scaling. Can also remove a small amount of iron.
Carbon Filter: Used to remove chlorine and organics from the feed water. Free chlorine will cause rapid irreversible damage to the membranes. The residual free chlorine present in most municipal water supplies will damage the thin film composite structure of the membranes used in this unit. Carbon filtration or sodium bisulfate injection should be used to completely remove the free chlorine residual.
Physical Conditioner: Under most conditioners in carwash feed water scale can be controlled through a physical conditioner.
Prefilter Cartridge: Used to remove smaller suspended solids and trap any particles that may be generated by the other pretreatment. The cartridge(s) should be replaced when the pressure drop across the housing increases 5-10 psig over the clean cartridge pressure drop. Typically installed on the Feed water side of the RO and not considered the primary pretreatment.
Iron and Manganese: These foulants should be removed to less than 0.3 ppm. Special media filters and/or chemical treatment is commonly used.
Silica: Reported on the analysis as SiO2. Silica forms a coating on membrane surfaces when the concentration exceeds its solubility. Additionally, the solubility is highly pH and temperature dependent. Silica fouling can be prevented with chemical injection and/or reduction in recovery. Providing the proper pre-treatment will extend the life of the membrane(s).
Reverse Osmosis in the carwashing industry
We are all aware of the value of RO product water in the carwashing industry. Rinsing with RO water, due to its lower mineral content, will reduce spotting on the vehicle. Reverse osmosis water displaces the mineral-heavy reclamation water or municipal water. This is especially true if the wash is located near a salt water area. RO water also enables a carwash operator to reduce the demands on the vehicle drying equipment such as air blowers. There are also other benefits to the use of RO water, such as lessening the need for towel drying after the vehicle has finished the wash process. By using RO water to mix soap, you also reduce the amount of soap used due to the fact that the RO water has fewer minerals and total dissolved solids (TDS) which will allow the soap to be more effective. Using RO water in express tunnel operations where no attendant is available will produce a better finished product than rinsing with well water or a municipal water source.
The water discussed in the above paragraph is known as the permeate water. This is the water which has passed through the membrane which in most cases will reduce the TDS by 98 percent. The other source of water produced from the RO process is called the concentrate or reject water. This is the water that has been rejected or did not pass through the membrane(s) of the system. This water will contain the minerals and TDS that was in the original source water.
The concentrate water can be saved for use in the wash process. This water can be used as a replacement for municipal water or well water in many applications. It is not recommended to be used for mixing of chemicals due to the high TDS levels, but can be a replacement for other functions that normally would require a freshwater source. One way the concentrate water is used is to capture it in a tank and use a pump to pressurize it to send to the wash process. You can then operate the processes in the wash that would normally use source water only with a combination of the sources mentioned above. With this set up you can drastically reduce the need to purchase water from a municipality, and using the concentrate water will also reduce the amount of water that is being sent to the sewer, another source of savings for you to consider.
You have already purchased concentrate water; you might as well put it to good use.

